A very
controversial paper was published 11 years ago in one of our most prestigious
medical journals by a group of prominent epidemiologists. In this thought provoking paper in the New England Journal of Medicine the
authors predicted a decline in life expectancy in the United States in this
century. Their words were disturbing, “…the
steady rise in life expectancy observed in the modern era may soon come to an
end and the youth of today may, on average, live less healthy and possibly
shorter lives than their parents”.
These
projections were based on the continuing increase in diseases driven mainly by
lifestyle factors such as nutrition and exercise. The most glaring example is the explosion in
type 2 diabetes with the percentage of the population with it doubling about
every 15 years. The part of this iceberg
not well seen as it is “below the water line” is prediabetes which now affects
1 in 3 U.S. adults and 15% of adolescents.
So back to
the part I never wanted to have to post.
The purpose of that controversial paper was to enlighten us about the
dead end that lay at the other end of the route through the maze that society
was taking with lifestyle. The
enlightenment was, of course, to push our lifestyle route through this maze in
another direction.
Unfortunately,
this message got ignored perhaps based on two other misleading messages. These are that high carb, sugar loaded,
chemical laden manufactured diets that the U.S. consumes couldn’t be that bad
and that no matter what we do to ourselves, there are drugs and medical
procedures that will serve as the wild card to negate the risk. We now know the message the prominent
epidemiologists gave us 11 years ago was accurate and that these other two competing
messages are largely untrue.
The National
Center for Health Statistics which tracts statistics on most aspects of our
nation’s health revealed some sobering data earlier this month. For the first time since 1993 during the peak
of the AIDs epidemic life expectancy in the U.S. declined. The current youngest children will have a
life expectancy less that of the parents and grandparents.
The primary
reasons for the developing decline are the increasing rates of deaths from
diseases including:
- Heart disease
- Stroke
- Diabetes
- Alzheimer’s disease
- Kidney disease
These
statistics are in spite of ever growing numbers of adults being treated with
drugs such as statins which are assumed to be preventing this outcome. Telling of this disconnect was shown in a
study published this year in the Journal
of the American Heart Association.
The study looked at the ability of statin treatment to prevent or
improve plaque build-up in the carotid artery, a factor that is known to
increase the risk of both stroke and heart disease.
While non-obese
subjects had an average plaque reduction of -4.2% after one
year of statin treatment, obese subjects had an average +4.8% increase with the
same treatment.
It seems
arterial disease is driven by the interaction of several factors not just the
levels of LDL cholesterol. One
noticeable associated factor was an inflammatory marker called C-reactive
protein (CRP). Central body fat (belly,
waist, hips) generates pronounced inflammation which increases the risk of all
5 diseases mentioned in the list above.
Elevated CRP increased the risk of plaque progression 156% in 1 year.
Before the
mind goes to just add an anti-inflammatory drug, a couple of things should be
considered. Their long-term use is associated
with substantial increased risk of renal failure which is one of the cited
diseases driving the downturn in longevity.
In contrast for every hour of sedentary time replaced by moderate
physical activity there is a 24% reduction in the inflammatory marker CRP.
There are
many more examples of the inferiority of the treatment of lifestyle driven
abnormalities with drugs versus corrective lifestyle. I talk to several prediabetics each month who
are not aware that they have prediabetes in spite of lab studies demonstrating
it for a couple of years. I also talk to
diabetics who have been told their blood HA1C levels are good at 7.0% because
the medication has lowered it from 9.5%.
The normal range is <5.7% and the increased vascular disease
risk at 9.5%, or poorly controlled diabetes, is +130%. While the risk is lower at an HA1C of 7.0% it
is still 40-60% higher than if it was in the normal range.
So why is
6.5%-7.0% which is the upper prediabetic range “good control” with medication?
Studies have shown that pushing it lower with that type of treatment will cause
episodes of intermittent hypoglycemia and actually increases overall death
risk. The only way to safely improve
more in that circumstance is with intense lifestyle management including
dietary change, exercise and weight loss.
Another example
of how these diseases interrelate was discussed in the scientific section of
the European Association for the Study of Diabetes. Dutch researchers reported their study of
brain changes associated both with diabetes and with prediabetes. The reason for looking at this is that
diabetes is a strong risk factor for developing some form of dementia such as
Alzheimer’s disease.
Two imaging
findings are associated with the brain changes driving dementia in diabetes. The first is diminished brain volume which
represents actual loss of large numbers of neurons or brain cells. The second is white matter lesions (WMLs)
which represent small areas of damage caused by altered blood flow.
Diabetics
had 167% greater numbers of WMLs than healthy controls. Most surprisingly, prediabetics demonstrated
considerable increased WMLs with 66% more than age comparable healthy adults.
Brain volume
reductions showed similar patterns with diabetics having the greatest but prediabetics
having abnormal amounts as well. The
structural brain changes associated with eventual dementia are present in
prediabetes but just not as advanced as in diabetes.
So the
circumstances at the time I wrote newsletter articles about the New England Journal of Medicine paper
11 years ago have changed. That alarming
projection has become an alarming reality.
What is the same is two-fold.
First is the 11-year old projection should serve as a dramatic wake-up
call. The second is the solution remains
the same although more urgent.
In the
developing years of 20th century healthcare infectious disease was a
major cause of death, and it could be effectively treated with a single drug. That idea has persisted as the main tenent of
health care. The 21st century
finds very different challenges, complex multi-system diseases highly related
to several interacting lifestyle errors.
These diseases are not well managed with the one disease/one drug
approach we have seemed to carry over.
They are also not ideally managed with the common 6-10 drugs that are
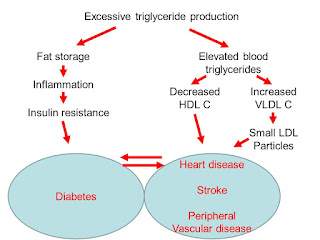
trying to get at late effects of chronic lifestyle neglect. We are working only in the right side of first
diagram above. The solution really lies
in working predominantly on the left side of it.
Olshansky et al. A POTENTIAL DECLINE IN LIFE EXPECTANCY IN THE
UNITED STATES IN THE 21ST CENTURY.
New England Journal of Medicine, 2007;352:1138-1144.
Sandfort et al. OBESITY IS ASSOCIATED WITH PROGRESSION OF
ATHEROSCLEROSIS DURING STATIN TREATMENT.
J Amer Heart Assoc, 2016;5:e003621.
Perneger et al. RISK OF KIDNEY FAILURE ASSOCIATED WITH THE
USE OF ACETAMINOPHEN, ASPRIN, AND NONSTERIODAL ANTIINFLAMMATORY DRUGS. New England Journal of Medicine, 1994;331:1675-1679.
Falconer et al. SEDENTARY TIME AND MARKERS OF INFLAMMATION IN
PEOPLE WITH NEWLY DIAGNOSED TYPE 2 DIABETES.
Nutrition, Metabolism & Cardiovascular Disease, 2014;24:956-962.
Sullivan MG. BRAIN ATROPHY IS ALREADY EVIDENT IN PATIENTS
WITH PREDIABETES. Clinical Endocrinology
News, Sept 14, 2016.